Abstract
Introduction: Fc gamma receptors (FCGR) are critical mediators of anti-CD20 monoclonal antibody-mediated cell-killing in lymphoma patients (pts). Previous studies have investigated the impact of FCGR genetics on pts' responses to treatment with rituximab (R). In particular, Single Nucleotide Polymorphisms (SNPs) in FCGR2A H131R (rs1801274), FCGR3A F158V (rs396991), and FCGR2B I232T (rs1050501) have been shown to affect either affinity for IgG (H131R; F158V) or receptor activity (I232T) (reviewed in Hargreaves et al. Immunol Rev 2015). Although the impact of these SNPs on effector function has been demonstrated in vitro, their influence on overall pt response is less clear, with multiple small cohort studies reporting inconsistent effects, likely due to their limited size. Therefore, we assessed the clinical importance of FCGR genotypes in two large, international, randomized Phase III clinical trials, assessing their potential impact on the efficacy of R or obinutuzumab (GA101; G) in combination with chemotherapy in pts with untreated advanced follicular lymphoma (FL) (GALLIUM; NCT01332968) and untreated diffuse large B-cell lymphoma (DLBCL) (GOYA; NCT01287741).
Methods: Genomic DNA was extracted from peripheral blood mononuclear cells in 2465 pts enrolled in GALLIUM (R-chemo vs G-chemo; n=1144 of 1202 enrolled) and GOYA (R-CHOP vs G-CHOP; n=1321 of 1418 enrolled). The key SNPs, FCGR2A H131R (rs1801274), FCGR3A F158V (rs396991), and FCGR2B I232T (rs1050501), were genotyped in all samples using TaqMan™ discrimination assays, with confirmatory Sanger sequencing employing FCGR-gene-specific polymerase chain reaction primers in 10% of cases. For both trials, our analysis of progression-free survival (PFS) included a univariate Cox regression analysis adjusted for treatment arm, and multivariate Cox regression analysis adjusted for stratification factors (GALLIUM: FLIPI and chemotherapy regimen; GOYA: IPI, cell of origin [COO], number of CHOP cycles, and BCL2;). In GOYA, we also adjusted for COO and BCL2 protein expression as we have previously shown these to be prognostically significant (Vitolo et al. J Clin Oncol 2017; McCord et al. Blood 2017). Multiple test correction was performed using Benjamini and Hochberg methodology.
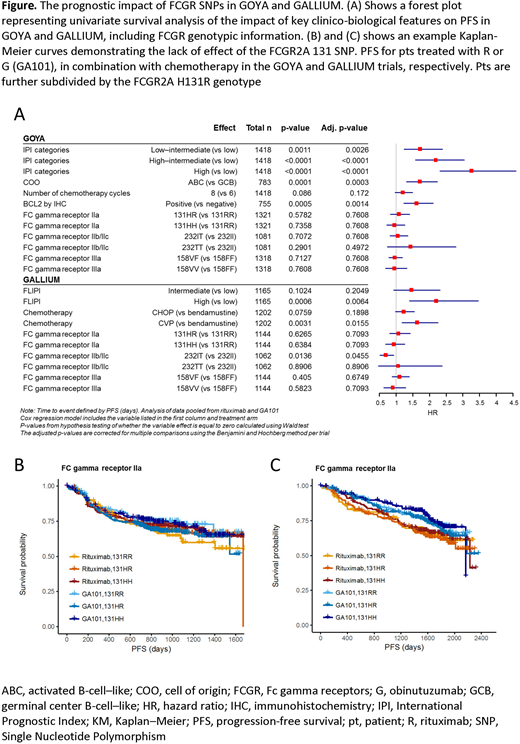
Results: The demographic and clinical characteristics of the FL and DLBCL pts included were comparable to those previously reported for the entire GALLIUM and GOYA trials, respectively. The prevalence of each FCGR genotype was comparable across trials, observed in both cohorts as: FCGR2A: R131R, 21.2% (523/2465 pts); H131R, 46.5% (1146/2465 pts); and H131H, 32.3% (796/2465 pts); FCGR3A: F158F, 43.6% (1073/2462); V158F, 44.5% (1096/2462); and V158V, 11.9% (293/2462); FCGR2B: I232I, 74.5% (1596/2143); I232T, 23.5% (503/2143); and T232T, 2.1% (44/2143). In FL, only FCGR2B was associated with PFS in univariate analyses comparing I232T to I232I genotype (Figure), with a more modest association for R treated pts (hazard ratio [HR] 0.78; 95% confidence interval [CI] 0.54─1.14; p=0.21) compared to G treated pts (HR 0.56; 95% CI 0.34─0.91; p=0.02). However, neither observation retained statistical significance in stratified analyses. In DLBCL, there was no evidence of a univariate prognostic effect of FCGR genotype. However, in multivariate analysis, we observed an association in the R treatment arm of GOYA with the FCGR2B T232T SNP (HR 4.37; 95% CI: 1.69-11.30; p=0.002 and multiple testing adjusted p=0.03) compared to the I232I pts. However, this observation should be interpreted with caution, given the low prevalence of this genotype (n=13) and limited number of PFS events (n=6).
Conclusions: We analyzed FCGR genotype status in 2465 pts with indolent and aggressive lymphoma treated with chemotherapy in combination with R or G and identified no clear prognostic impact of the three key FCGR SNPs. In conclusion, this study, the largest performed to date, provides further conclusive evidence that FCGR genotype does not confer differential responsiveness to R or G in treatment naive pts with advanced FL or aggressive DLBCL.
JCS, MN and CH share first authorship, MCC and MZO share senior authorship.
Strefford:University of Southampton: Employment; F.Hoffman-La Roche: Research Funding. Nowicka:F.Hoffman-La Roche: Employment. Parker:Kay Kendall Leukaemia Fund: Research Funding; University of Southampton: Employment. Knapp:Roche: Employment. Mir:F.Hoffman-La Roche Ltd: Employment. Rose-Zerilli:Leuka Charity: Research Funding. Burton:NHS: Employment; Takeda: Honoraria; Roche: Honoraria; Takeda: Consultancy; Roche: Consultancy; Bristol-Myers Squibb: Consultancy. Hiddemann:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Consultancy, Research Funding; F. Hoffman-La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Klapper:HTG Molecular Diagnostics, Inc.: Research Funding; Regeneron: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; F.Hoffman-La Roche: Honoraria, Research Funding. Sehn:Morphosys: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Merck: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria; Lundbeck: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Roche/Genentech: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria. Vitolo:Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Gilead: Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Sandoz: Speakers Bureau; Takeda: Speakers Bureau; Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Martelli:Celgene: Membership on an entity's Board of Directors or advisory committees; Sandoz: Membership on an entity's Board of Directors or advisory committees; Mundipharma: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Membership on an entity's Board of Directors or advisory committees; F. Hoffman-La Roche: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees. Trněný:Morphosys: Membership on an entity's Board of Directors or advisory committees, Other: Advisory board; F. Hoffman-La Roche Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisory board, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Advisory board; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisory board; Sandoz: Honoraria; Abbvie: Honoraria, Research Funding; Gilead: Honoraria; Incyte: Membership on an entity's Board of Directors or advisory committees, Other: Advisory board; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Advisory board. Davies:F. Hoffman-La Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Pfizer: Research Funding; Acerta Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Research Funding; Kite: Consultancy; GSK: Research Funding; Janssen: Consultancy, Honoraria; Gilead: Honoraria, Research Funding; Karyopharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Cragg:Bioinvent: Consultancy, Patents & Royalties: Patent licenced to Bioinvent around CD32b blockade; Boehringer Ingleheim: Consultancy. Oestergaard:Roche: Employment, Other: Ownership interests PLC.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal